The
Lear Laboratory
Read what we have done
2020

We explore the oxygen stability of chalcogen-metal interfaces for small nanoparticles and find that, though Se is susceptible to oxidation for Au particles, Se provides a stable interface for Ir and Pt particles.
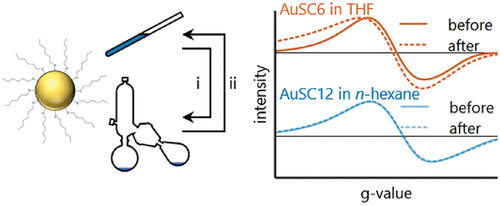
Dependence of Core Electronics of Gold Nanoparticles on Ligand, Solvent, and Sample Preparation
Using ESR spectroscopy as a probe of the electronic properties nanoparticle metallic cores, we explore how manipulations of these particles---such as freeze/thaw cycles---perturb the the core for dispersions of alkanethiolate-protected gold nanoparticles in organic solvents.

Nanoscale heat for organic transformations: a photothermally driven retro-Diels-Alder reaction
We show that conventional organic reaction can be driven using photothermal heating. Even in low boiling point solvents, such as dichloromethane, the localized heat provided by photothermal heating drive the reactions at rates associated with extreme temperatures.
2019

Structural and Solvent control over activation parameters for a pair of retro Diels-Alder reactions
A significant amount of work has been devoted to understanding the parmameters that govern the kinetics of Diels-Alder reactions. Less work has been directed towards the retro-reaction. Here, we examine the rate of cycloreversion for a series of Diels-Alder adducts, and examine the dependence of activiation parameters on the adducts and solvent.

Photothermal control over mechanical and physical properties of polydimethylsiloxane
We show that conventional organic reaction can be driven using photothermal heating. Even in low boiling point solvents, such as dichloromethane, the localized heat provided by photothermal heating drive the reactions at rates associated with extreme temperatures.

Controlled Rapid formation of polyurethane at 700 K: Thermodynamic and kinetic consequences of extreme heating
Photothermal heating has already been shown by ourselves and others to drive reactions at rate that imply extreme temperatures. By following a reaction to the point of equilibrium, we show that the thermodynamics of the systems also reflect this temperature. However, despite highly elevated temperatures, the chemical reaction under consideration shows no signs of thermolysis.
2018

Photothermal effectiveness of magnetite nanoparticles: Dependence on particle size probed by experiment and simulation
We show that conventional organic reaction can be driven using photothermal heating. Even in low boiling point solvents, such as dichloromethane, the localized heat provided by photothermal heating drive the reactions at rates associated with extreme temperatures.
2017

On-demand curing of polydimethylsiloxane (PDMS) using the phottohermal effect of gold nanoparticles
We show that conventional organic reaction can be driven using photothermal heating. Even in low boiling point solvents, such as dichloromethane, the localized heat provided by photothermal heating drive the reactions at rates associated with extreme temperatures.
2016

Chain length and solvent control over the electronic properties of alkanethiolate-protected gold nanoparticles at the molecule-to-metal transition
Using electron spin paramagnetic resonance to probe the metallic electrons of gold nanoparticles, we show that changes to the ligand identity, as subtle as changes in alkanethiolate length, controls the position of the Fermi energy for the metallic core.

Probing ligand-induced modulation of metallic states in small gold nanoparticles using conduction electron spin resonance
Read at: Physical Chemistry Chemical Physics, 18, 25443
Examining the behavior of metallic electrons in the core of metallic nanoparticles, we show that the identity of para-substituted aromatic thiolates can be used to control the Fermi energy of the core. We also show that the Hammett parameter of the ligand correlates with the observed EPR behavior.

Steady-State Spectroscopic Analysis of Proton-Dependent Electron Transfer on Pyrazine-Appended Metal Dithiolenes [Ni(pdt)2], [Pd(pdt)2], and [Pt(pdt)2] (pdt = 2,3-Pyrazinedithiol)
Read at: Inorganic Chemistry, 55, 8459
For a series of dithiolene-based mixed valence complexes, we show that the asymmetric protonation produces charge pinning of the unpaired electron, and significant reduction in electronic coupling.

Effect of Protonation upon Electronic Coupling in the Mixed Valence and Mixed Protonated Complex, [Ni(2,3-pyrazinedithiol)2]
Read at: Inorganic Chemistry, 55, 1433
For a single pyrazinedithiol complex, we demonstrate that asymmetric protonation of the mixed valence state produces profound changes in the electronic coupling.

Synthesis and characterization of the gold dithiolene monoanion, (Bu4N)[Au(pdt = 2,3-pyrazinedithiol)2]
Read at: Polyhedron, 103, 100
We examine the electrochemical and acid-base properties of a pyrazinedithiol gold complex.
2015

Isolation and Chemical Transformations Involving a Reactive Intermediate of MOF-5
MOF-5 is one of the most common and stable metal-organic frameworks. Here, we demonstrate an intermediate obtained during the synthesis of MOF-5 is reactive, and can be transformed into other metal-bearing MOFs via a solid state transformation.

Ligand Control over the Electronic Properties within the Metallic Core of Gold Nanoparticles
Read at: Angewante Chemie, International Edition, 54, 11750
Ligands are a ubiquitous feature of non-acqueous gold nanoparticles, and are often used to control their stability and interaction with the environment. Using ESR, we demonstrate that choice of ligand also influences the properties of the metal core.

Billion-fold rate enhancement of urethane polymerization via the photothermal effect of plasmonic gold nanoparticles
Read at: Chemical Science, 6, 6462
We demonstrate that the photothermal effect of nanoparticles can be used to drive the thermal curing of polyurethane at extreme rates. We obtain efficient bond formation, despite using reactive temperatures in excess of 600 K.

Structural, Electronic, and Magnetic Characterization of a Dinuclear Zinc Complex Containing TCNQ– and a μ-[TCNQ–TCNQ]2– Ligand
Read at: Inorganic Chemistry, 54, 6072
TCNQ is valued for its stability as a stable radical. Here, we show that coordination with Zinc increases the reactivity of TCNQ to th epoint that it forms a homodimer.

Comparing the Energetic and Dynamic Contributions of Solvent to Very Low Barrier Isomerization Using Dynamic Steady-State Vibrational Spectroscopy
Read at: The Journal of Physical Chemistry A, 119, 3545
Using spectral broadening to report on ground state dynamics, we examine the effects of solvent on the turnstile isomerizatoin of a pianostool-type carbonyl complex, uncovering the contributions of both solvent dynamics and energetics to such ultrafast processes.

Fe3O4 nanoparticles as robust photothermal agents for driving high barrier reactions under ambient conditions
Read at: Chemical Communications, 51, 417
The photothermal effect of nanoparticles can be used to drive chemical reactions at extreme rates, but can also lead to drastic changes in the nanoparticles used for the effect. Here, we show that the size, shape, and even surface chemistry of iron oxide particles are stable under even the most aggressive photothermal conditions.